The University of Nottingham
 Exchange online
Exchange online
Research Exchange
Mutant parasite could stop malaria in its tracks
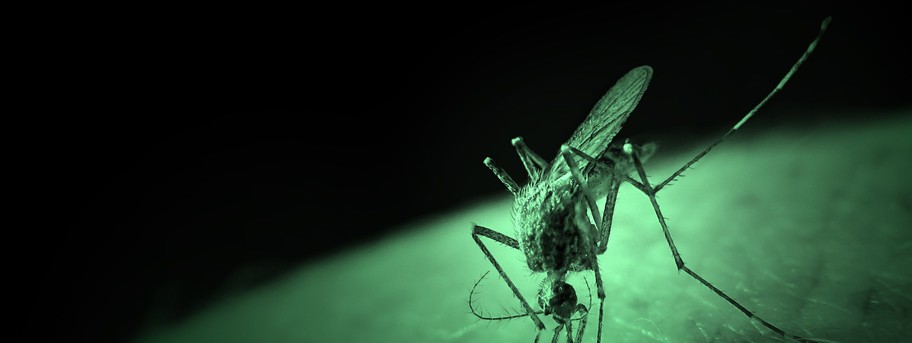
University of Nottingham malaria experts have found a way of disabling one of the many phosphatase proteins which breathe life into the malaria parasite. The result is a mutant which is unable to complete the complex life cycle crucial to its development. The discovery could help to design drugs to save thousands of lives.
The research led by Dr Rita Tewari in the Centre for Genetics and Genomics in the School of Biology in collaboration with the University of Oxford, Imperial College London, the University of Leicester and the MRC National Institute for Medical Research, has been published in the prestigious open access journal PLOS Pathogens.
The researchers, funded by the MRC and the Wellcome Trust, have worked out how the unique enzyme — PPKL phosphatase — controls the development of the parasite at an essential stage for transmission. By removing this enzyme other proteins fail to work properly and the resulting mutant has the wrong shape to burrow through the stomach wall of the female Anopheles mosquito and pass on the disease to humans.
Approximately 40 phosphatase enzymes are present in the genome of malaria parasite — PPKL is the first whose function is identified. The research group has already begun work on identifying the rest.
Dr Tewari said: “This is the first step in understanding the functional role of phosphatases in malaria. This enzyme is absent in humans and so it can be explored as a good target for malaria control and transmission. The control of parasite transmission is important in order to prevent the spread of malaria. Targeting PPKL can be an important player in this process.”
The life of a malaria parasite
Malaria is spread by transmission by the female Anopheles mosquito. The life cycle of the malaria parasite is complex. The sporozoite form of the parasite is injected into the human blood stream with mosquito saliva. It then takes just 30 minutes for the sporozoites to find and enter liver cells. Within five to seven days they have developed into thousands of merozoites. These merozoites burst out of the liver, into the blood stream and invade red blood cells where the parasite multiplies again. Two days later new merozoites burst from the blood cells and infect more blood cells. Some merozoites develop into gametocytes — the sexual stages of the parasite — and these are taken up by another mosquito while it is taking its blood meal. Inside the mosquito gut the gametocytes develop into gametes and fuse to form a zygote. It is at this stage — when the zygote is transforming into a mobile ookinete — that the scientists can strike.
Breaking the complex life cycle
The banana shape of the ookinete gives it a special tip so it can invade the gut wall. The research group has shown that the abnormal ookinetes have lost their ‘banana’ shape and all their essential functions. If it can’t break through the gut wall the transmission of malaria is stopped.
Tony Holder, Head of Parasitology at the MRC National Institute for Medical Research UK and a senior collaborator in the study, said: “Transmission through the mosquito represents a bottle neck in the parasite’s life cycle. Intervention strategies to target these stages will be essential for the long term goal to eradicate and finally eliminate malaria.”
The three main research scientists in the Nottingham group are Dr David Guttery, Dr Benoit Poulin and Dr Eva Patzewitz.
The full research paper is available here.
Tags: Centre for Genetics and GEnomics, malaria, mosquito, MRC National Institute for Medical Research, parasite, School of Biology
One Comment
Leave a Reply
Other News

Top prize for quantum physicist
A University of Nottingham physicist has won a prestigious medal from the Institute of Physics for […]

Zero carbon HOUSE designed and built by students comes home
Design and construct a low cost, zero carbon, family starter home, transport it to Spain, build […]
October 5th, 2012 at 5:51 am
Scrutinizing a Killer
[…] found on Otzi the icemanMicrocirculation Of Malaria-Infected Red Blood Cells Impeded By ProteinMutant parasite could stop malaria in its tracks – Research, The University of Nottingham .wp-pagenavi { font-size:12px !important; } […]